GI-Cell opens the way for autoimmune disease treatments by obtaining patent for regulatory T cell large-scale culture technology Patent to enhance GI-Cell’s competitiveness in autoimmune disease treatments
공개 2021-11-30 08:14:15
이 기사는 2021년 11월 30일 08:06 thebell 에 표출된 기사입니다.
GI-Cell, a South Korea-based company specializing in immune cell therapy, announced on November 29 that it obtained a domestic patent for a large-scale culture technology of regulatory T cells for the treatment of autoimmune diseases. With this patent, GI-Cell is expected to become a leader in the autoimmune disease space in the country.Regulatory T cells maintain immune system homeostasis through immunosuppression regulation. Regulatory T cells have been in the spotlight as a therapeutic agent for diseases caused by imbalance of the immune system, such as autoimmune diseases. However, the proportion of regulatory T cells among the total T cells is only about 2-3%, making it difficult to separate and culture cells. It is also difficult to develop therapeutic agents because the characteristic properties of regulatory T cells are easily lost during the culturing process. However, GI-Cell succeeded in maintaining highly functional regulatory T cells through its unique and differentiated culture technology with improved productivity.
FoxP3, a key marker that can confirm the function of regulatory T cells, needs to be continuously expressed during cell culture. GI-Cell confirmed that FoxP3 expression was maintained in almost all cells even after the culture was finished, and the regulatory T cells were successfully cultured up to tens of billions of cells in the research stage. In addition, the regulatory T cells under development at GI-Cell have regulated surface receptor expression to target specific organs or inflammatory lesions (Code name GIC302, Brand name Drone Treg), and can more effectively suppress the inflammatory response within the lesion.
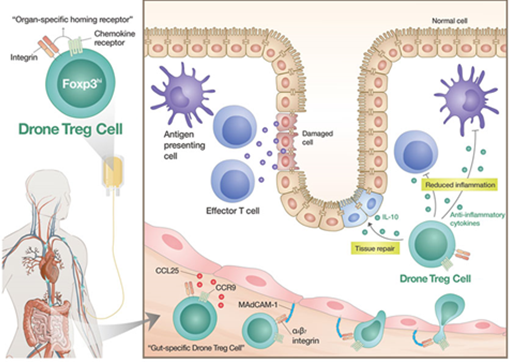
“This patent is recognized for overcoming the limitations of existing regulatory T cell production technology by manufacturing a large amounts of highly active regulatory T cells through a proprietary culture ancillary material and manufacturing methods without changing any characteristic properties of the cells,” said the inventors of the patent, GI Group Chairman Dr. Myoung Ho Jang and GI-Cell CEO Dr. Chun Pyo Hong. “By utilizing Drone Treg platform technology, GI-Cell will spur the development of cell therapies for various autoimmune diseases.”
GI-Cell was recognized in March as an excellent company in job invention compensation by the Korean Intellectual Property Office. This enabled the company to apply for an expedited examination process and register the patent quickly.

< 저작권자 ⓒ 자본시장 미디어 'thebell', 무단 전재, 재배포 및 AI학습 이용 금지 >
best clicks
최신뉴스 in 전체기사
-
- 제4인터넷은행의 운명은
- 더케이저축, 부동산 대출서 '부실'…공제회에 손 벌릴까
- [삼성 보험 신체제 1년 점검]삼성화재, 초격차 성과 만들어낸 '볼륨 확대' 전략
- [농협금융 인사 풍향계]NH농협캐피탈 대표에 장종환 본부장…영업 채널 다각화 과제
- [농협금융 인사 풍향계]농협손보 대표에 '보험통' 송춘수…첫 내부출신 수장
- [농협금융 인사 풍향계]5곳에서 대표 교체…강호동 회장 '쇄신' 선택
- [농협금융 인사 풍향계]NH저축, 1년 만 대표 교체…'금융통' 김장섭 낙점
- [우리금융 인사 풍향계]우리금융캐피탈 대표에 기동호…IB·기업금융 고삐 죈다
- [농협금융 인사 풍향계]1년 만에 돌아온 강태영 행장 후보, 내부통제 강화 과제
- [DGB금융 인사 풍향계]황병우 회장, iM뱅크 은행장 겸직 이어간다




















